Long non‑coding RNAs (lncRNAs) play a critical role in tumorigenesis. LncRNAs can be classified into tumor suppressor genes or oncogenes, depending on their function within the cellular context and the signaling pathways in which they are involved. These regulatory RNAs are potential therapeutic targets for cancer due to their tissue and tumor specificity. The lncRNA Mortal Obligate RNA Transcript (MORT; alias ZNF667-AS1) has been identified as a tumor-related lncRNA.
This new review paper by Di Fiore, R et al. is entitled ‘LncRNA MORT (ZNF667-AS1) in Cancer – is there a possible role in Gynecological Malignancies?'. This publication has been carried out in collaboration with 14 co-authors hailing from 6 different countries, as part of the GYNOCARE COST Action (CA18117). In this review, there is a description of the biological and regulatory functions of ZNF667-AS1 in human disease, including cancer. Furthermore, emerging insights into the potential role of ZNF667-AS1 as a biomarker and novel therapeutic target particularly in gynecological cancers, are explored.
This work has also received a special mention by the Sbarro Health Research Organisation, Temple University, Philadelphia, USA.
Publication
Di Fiore R, Suleiman S, Drago-Ferrante R, Felix A, O’Toole SA, O’Leary JJ, Ward MP, Beirne J, Yordanov A, Vasileva-Slaveva M, Subbannayya Y, Pentimalli F, Giordano A, Calleja-Agius J. LncRNA MORT (ZNF667-AS1) in Cancer—Is There a Possible Role in Gynecological Malignancies? International Journal of Molecular Sciences. 2021; 22(15):7829. https://doi.org/10.3390/ijms22157829
Click here to read the full text.
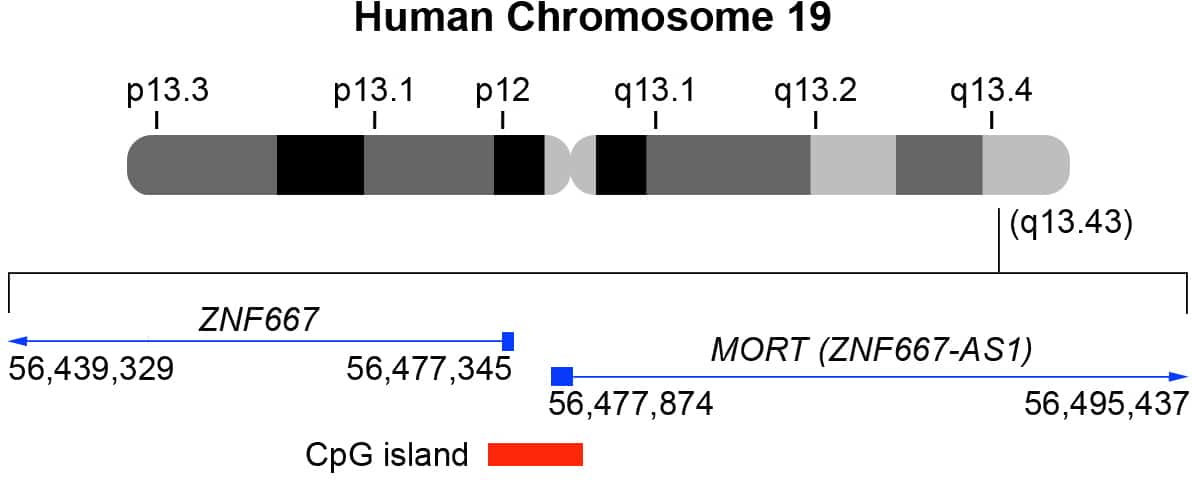
MORT (ZNF667-AS1) and ZNF667 genomic location
MORT (ZNF667-AS1) and ZNF667 are head-to-head antisense-sense strands. MORT (ZNF667-AS1) is also located in 19q13.43 (GRCh38p13 database, chr19: 56,477,874-56,495,437; NCBI: NR_036521.1). ZNF667 is also located in 19q13.43 (GRCh38p13 database, chr19: 56,439,329-56,477,345; NCBI: NM_022103.4).
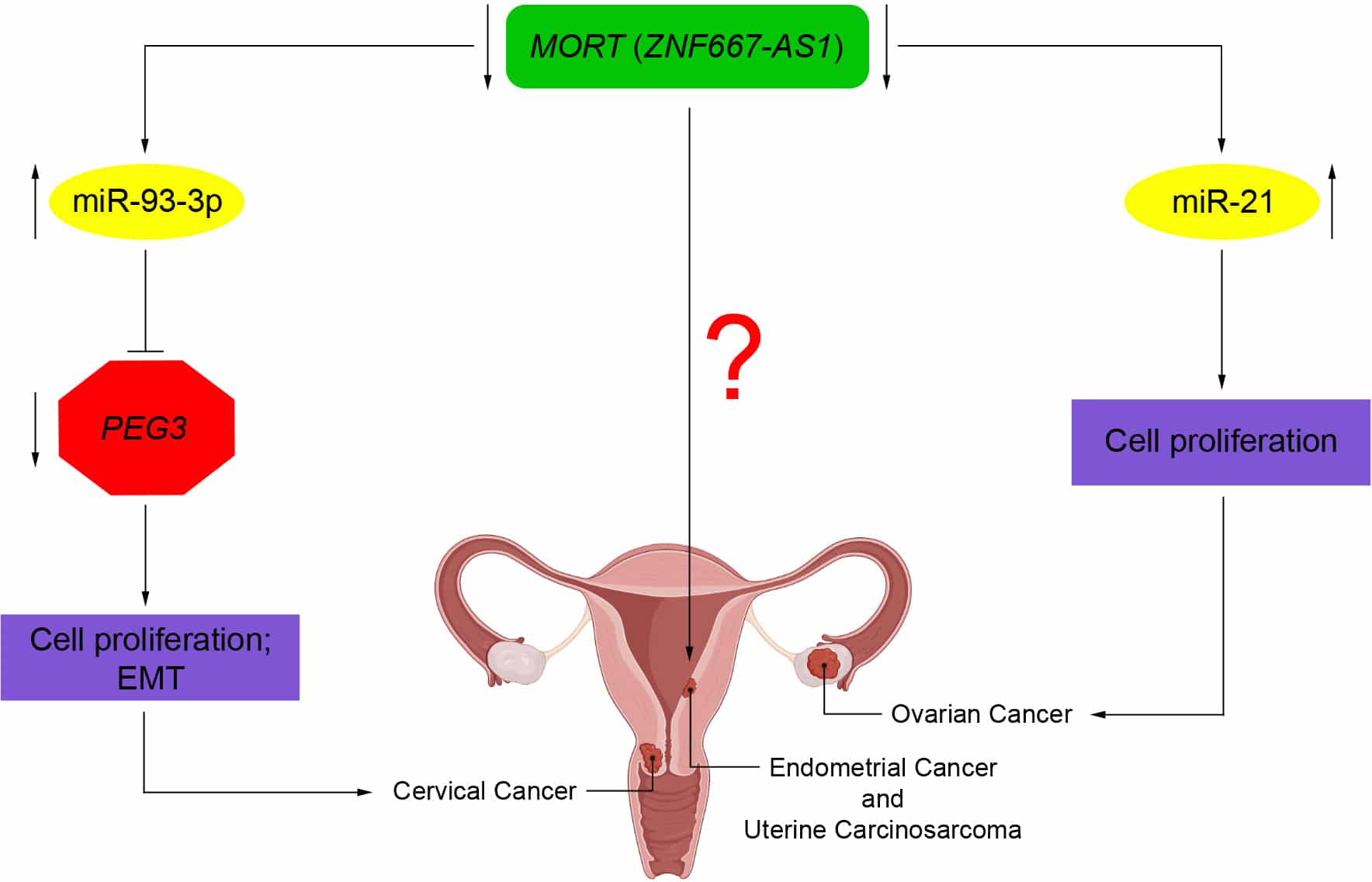
Pathological role of MORT (ZNF667-AS1) in gynaecological cancers
Underexpression of MORT (ZNF667-AS1) could promote tumor initiation in ovarian, cervical, and endometrial cancers through different molecular mechanisms not yet well explored. In ovarian cancer, MORT (ZNF667-AS1) downregulation and consequent miR-21 overexpression promote the proliferation of ovarian cancer cells. In cervical cancer, downregulation of MORT (ZNF667-AS1) and consequent overexpression of miR-93-3p reduce the expression of PEG3, thereby allowing cell proliferation and EMT. In endometrial cancer and uterine carcinosarcoma, MORT (ZNF667-AS1) expression is silenced by aberrant DNA methylation. However, its function mechanism in these types of cancer is unknown.